Which of the Following Statements Correctly Describe a Redox Reaction
Which of the following statements about oxidation-reduction redox reactions is correct. Select all the statements that correctly describe the following redox reaction which is given as a molecular equation.
What Is A Redox Reaction Explain With An Example
Tagged with chemistry redox.

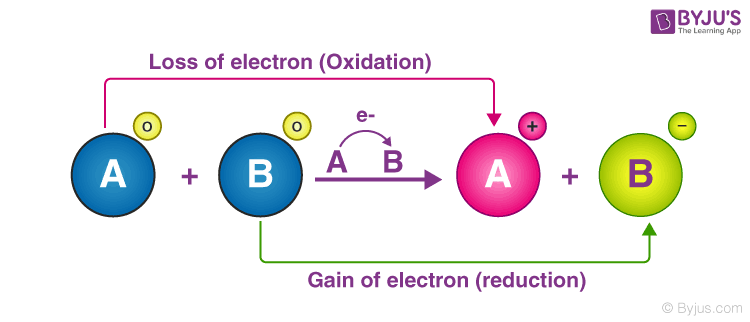
. The reactants are insoluble precipitates This type of reaction usually involves ionic compounds. Oxidation is the part of a redox reaction in which there is a loss of electrons by an atom. C H is the oxidizing agent.
A redox reaction is called like that because it involves a substance that is reducing and a substance that is oxidating so it is Re-dox this means that all of the chemicals reactions that involve a substance that looses an electron are redox reactions they are present in almost all of chemistry from syntetic to biological chemistry so the only correct option would be. In atmosphere formation of O 3. Select all the statements that correctly describe the following redox reaction which is given as a molecular equation.
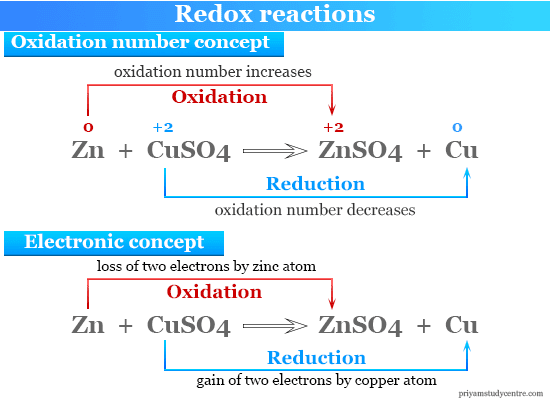
B Mg is oxidized to Mg2. As is implied by the name such a reaction has two parts and each part tells you what happens to the electrons. A NAD H 2e- NADH b C 6 H 12 O 6 6O 2 6CO 2 6H 2 O c Ca.
- In a Redox Reaction the oxidation states of the constituents should change. Mg s 2HCI aq Mgc2aH2 g Check all that apply. B It is unrelated to the free energy of the reaction.
Reduction is the part of a redox reaction in which there is a gain of electrons by an atom. Mg2 is the reducing agent. The electrons should cancel out leaving a balanced complete redox reaction.
H 2 SO 4 2NaOHNa 2 SO 4 2H 2 O This is a neutralisation reaction. H is reduced to H2. C It can be used to predict whether a given compound can reduce another.
Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids class 12 Atoms Chemical Kinetics Moving Charges and Magnetism Microbes in Human Welfare Semiconductor Electronics. Which of the following is a redox reaction. A The loss or donation.
What term is used to describe NAD in this context. It is correctly balanced for charge but not. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative elements.
It is correctly balanced. A The molecule that is oxidized loses electrons and is the oxidizing agent. Which of the following statements correctly describe a total ionic equation.
Select ALL that are correct. B The molecule that is reduced gains electrons and is the oxidizing agent. A H 2 SO 4 with NaOH B In atmosphere O 3 from O 2 by lightning C Nitrogen oxides form nitrogen and oxygen by lightning D Evaporation of H 2 O Medium Solution Verified by Toppr Correct option is C A.
Which of the following options correctly describes a. Select All The Options That Correctly Describe How Energy Is Involved In Chemical Reactions. Posted on January 9 2021 by January 9 2021 by.
Mg is oxidized to Mg. It is a series of redox reactions Requires mitochondria The method yeast use to produce ATP Pyruvate Oxidation is one of the steps Glycolysis is one of the steps Approximately 30 ATP molecules are produced Citric Acid Cycle is one of the steps Produces. D Mg2 is the reducing agent.
Produces carbon dioxide The method plants and animals rely on to produce large amount of ATP Glycolysis is one of the steps Oxygen is the final electron acceptor Approximately 30 ATP molecules are produced 0 It is a redox reaction Produces heat Requires. Select all that apply. C The molecule that is oxidized gains electrons and is the reducing agent.
D The total ionic equation includes all ions including spectator ions. The reaction forms a salt and water. Select all the statements that correctly describe the following redox reaction which is represented by the molecular equation below.
Which of the following options correctly describe the products of the reaction between a strong acid and a strong base in aqueous solutions. Think so I know it. H is the oxidizing agent.
Select all that apply. The name also implies that in any reaction in which oxidation occurs reduction must take. Select ALL that are correct.
Select all the options that correctly describe redox reactions. A It is used to describe phosphate group transfers. It is correctly balanced for the number of atoms but not for charge.
B Charges and atoms must be balanced in this type of equation. Mg s 2HCl aq MgCl2 aq H2 g a Cl- is reduced to Cl22-. Cl-is reduced to Cl Do you know the answer.
Materials Devices and Simple Circuits. A covalent bond in which the shared electron pair is distributed unevenly between the atoms. C This equation shows only the ions involved in a reaction.
Add the two half-reactions together. E H is reduced to H2. Which of the following statements correctly describe aerobic respiration.
Produces heat Oxygen is the final electron acceptor The method yeast use to produce ATP Oxidative phosphorylation is one of the steps Requires oxygen It is a series of redox reactions Citric Acid Cycle is one of the steps Pyruvate Oxidation is one. A Prosthetic group b Coenzyme c Functional group d Intermediate Question 6 Which of the following is an example of a redox reaction. A Charges must be included where appropriate in this type of equation.
Which of the following statements about the redox potential for a reaction are correct. Ni is oxidized to Ni2 Cl is oxidized. Select ALL that are correct.
B Mg is oxidized to Mg2. Ni is the oxidizing agent. Which of the following statements correctly describes the process of oxidation.
HCl aq Ni s KCr 0 aq KCl aq CrCl aq Nici aq H20 1 Check all that apply. Which of the following statements correctly describe aerobic respiration. Which of the following statements correctly describe fermentation.
Which of the following options correctly defines a polar covalent bond.

Redox Reactions Examples Types Applications Balancing
Redox Reactions Definition Types Examples Application

Question Video Describing The Redox Reaction Between Zn And Ag Using An Ionic Equation Nagwa
0 Response to "Which of the Following Statements Correctly Describe a Redox Reaction"
Post a Comment